HSV1, HSV2, VZV R-GENE®
Real-time PCR kit for the detection and quantification of Herpes Simplex types 1 & 2
Get accurate detection and quantification of Herpes Simplex types 1 (HSV-1) and 2 (HSV-2) as well as Varicella Zoster Virus (VZV) DNA with the HSV1 HSV2 VZV R-GENE® real-time PCR kit
- Accurate quantification of HSV-1, HSV-2 and VZV viral load over a wide linear range
- Ready-to-use kit that includes internal control and quantification standards
- CE-IVD on all major extraction platforms and real-time PCR systems and on different samples types
Do you need more information
HSV1, HSV2, VZV R-GENE® advantages
HSV-1, HSV-2 and VZV are responsible for a wide spectrum of clinical conditions from rashes to encephalitis in both immunocompetent and immunocompromised people. That’s why you want optimized detection and monitoring of HSV-1, HSV-2 and VZV infection. HSV1 HSV2 VZV virus R-GENE® is an ideal solution, offering rapid and specific detection. You can detect infection prior to clinical symptoms to improve options for management and test patients during treatment to measure its effectiveness. You can also test after treatment to monitor for relapse. For even more comprehensive testing, you can use the R-GENE® range to quantify various viruses from one sample or simultaneously analyze various samples for a single type of virus.
- Sensitive and reproducible
- Reliable measurement of HSV-1, HSV-2, VZV infection
- Wide linear range
- Standardized
- Parallel processing with R-GENE® range of products (CMV R-GENE®, Adenovirus R-GENE®, CMV HHV6,7,8 R-GENE®, BK Virus R-GENE®, EBV R-GENE®, Parvovirus B19 R-GENE®)
- Harmonized test profiles for multiple assays in one run
- Flexible
- Validated for use with various samples types
- Prepare samples manually or use qualified automated sample preparation such as NucliSENS® easyMAG® and assay setup systems such as easySTREAMTM liquid handling platform
- Qualified with the major real-time PCR platforms
Everything in just one kit
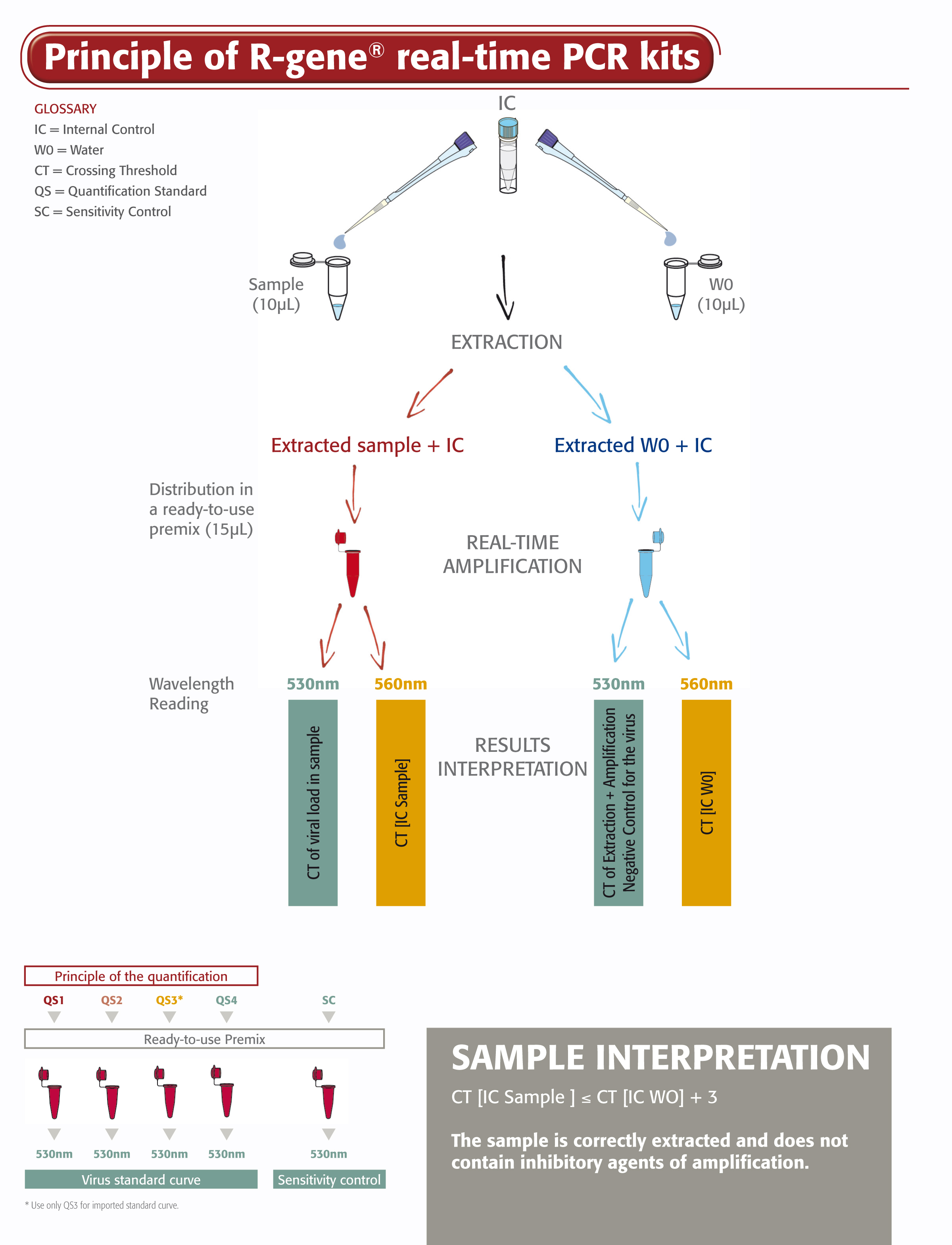
The HSV1 HSV2 VZV R-GENE® kit is a ready-to-use molecular detection kit that measures the viral load of HSV-1, HSV-2 and VZV using real-time PCR after viral DNA extraction. This 5’ nuclease-based real-time PCR assay amplifies a specific region of each virus genome.
- Four Quantification Standards ensure accurate HSV-1, HSV-2, VZV viral load measurement
- Sensitivity Control validates the performance of the assay
- An Internal Control (IC2) checks the extraction process, including lysis, and the presence of amplification inhibitors in the sample
- Includes all necessary reagents optimized to detect and quantify HSV-1 (60 tests), HSV-2 (60 tests) and VZV (60 tests) for in vitro diagnostic use
- Supplementary reagents also available: amplification premixes HSV1 R-GENE® (71-015 – 60 tests), HSV2 R-GENE® (71-016 - 60 tests), VZV R-GENE® (69-100R8 - 60 tests).
Simple procedure
It is simple to use the HSV1 HSV2 VZV R-GENE® kit. All you do is add the extracted DNA sample to the ready-to-use PCR master mixes and start the reaction on the appropriate Real-Time PCR thermocycler, following optimized cycling program described in the “Instructions For Use”.
Click to enlarge »
BIOMERIEUX, the blue logo, ARGENE®, R-GENE®, EASYMAG® and NUCLISENS® are used, pending and/or registered trademarks belonging to bioMérieux, or one of its subsidiaries, or one of its companies. Any other name or trademark is the property of its respective owner.
| Technical Specifications for HSV1 HSV2 VZV R-GENE® (69-004B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of HSV-1, HSV-2 and VZV |
| Ordering information | Reference 69-004B: HSV1 HSV2 VZV R-GENE® Quantification kit Also available under reference 69-004: HSV1 HSV2 VZV R-GENE® Quantification COMPLETE kit (include 69-004B Quantification kit and ref. 67-000 DNA extraction kit) Supplementary reagents: 71-015: HSV1 R-GENE® 71-016: HSV-2 R-GENE® 71-017: VZV R-GENE® |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| Gene target | HSV-1 : US7 gene HSV-2 : US2 gene VZV : gene coding for gp19 (ORF 17) |
| Specimen* | CSF, Ears Nose Throat (ENT) samples, Ophtalmologic samples, BAL, Plasma, Gynaecological smears, Cutaneous and mucous smears, Cell Culture |
| Limit of Detection | HSV-1: 2 Copies/ PCR HSV-2: 2 Copies/ PCR VZV: 2 Copies/ PCR |
| Dynamic Range of Quantification | HSV-1: Up to 107 copies/ mL HSV-2: Up to 107 copies/ mL VZV: Up to 107 copies/ mL |
| Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL |
| Number of tests | 180 tests |
| Storage conditions | -18°C/-22°C for reference 69-004B (Quantification kit), +2°C/+ |
| +8°C for ref. 67-000 (DNA extraction kit) | |
| Validated Extraction platform* Manual Automated | QIAamp DNA Blood Mini kit QIAamp MinElute Virus Spin kit QIAcube NucliSENS® easyMAG® MagNA Pure Compact MagNA Pure LC VERSANT kPCR Molecular System SP |
| Validated Amplification platform* | Applied Biosystems LightCycler SmartCycler RotorGene VERSANT kPCR Molecular System AD DX Real-Time system Stratagene / Agilent |
| Status | For in vitro diagnostic use, CE marking in Europe: Please inquire USA: For research use only, not for use in diagnostic procedure (ref.69-004B-01 and also 71-015-01, 71-016-01, 71-017-01) |
* please enquire
Fast facts on HSV-1, HSV-2 and VZV
What are HSV-1, HSV-2 and VZV?
There are eight human Herpesviridae, of which Herpes Simplex Virus Types 1 (HSV-1) and 2 (HSV-2) and Varicella Zoster Virus (VZV) are the most common in immunocompetent patients. The primary infection of HSV-1 and HSV-2 and VZV is generally limited to the mucous membranes and the skin. Usually benign, the infections linked with these viruses can nevertheless develop into severe clinical forms such as encephalitis, meningitis, retinitis and neonatal infections. After primary infection, the viruses persist in the host in a latent state. However, when assisted by chronic or transient immunosuppression, they can be reactivated, causing recurrent infections. In these severe and often atypical infections, the implicated virus cannot be identified. For many years various antivirals have proven their worth in efficiently treating these pathologies if prescribed early and at appropriate doses.
Who is most at risk?
Both immunocompetent and immunodepressed people can be affected by these viruses. Among the severe forms of HSV-1, HSV-2 or VZV infections in adults, HSV-1-induced encephalitis – of which there are 1/250,000 to 1/1,000,000 cases per year – can still be fatal if untreated. Encephalitis caused by HSV-2 or VZV usually has a more favorable prognosis. However, neonatal encephalitis can be traced to both HSV-1 and HSV-2 viruses, but the HSV-2 virus is responsible for the most serious neurological disorders. VZV virus has long been associated with encephalitis in immunodepressed patients. More recently, viral genome detection techniques have also increasingly shown VZV in meningo-encephalitis type infections in immunocompetent subjects.
What are the benefits of HSV-1, HSV-2 and VZV testing?
Conventional immunological culture and detection techniques are suitable for diagnosing the benign skin infections for which these viruses are responsible. However, they are unsuitable for severe infections of the central nervous system (CNS) and congenital infections. In severe infections, it is essential to obtain an early and rapid diagnosis of the infection. Quantitative HSV-1, HSV-2 and VZV DNA PCR tests are the ideal tool for CNS specimens and to follow congenital infections. Real-Time quantitative PCR-based assays for HSV1, HSV2 and VZV can be used to detect the presence of the virus and keep track of the course of the infections by monitoring the effectiveness of active treatment and for relapse after treatment.
Publications and Posters
- Measurement of HSV1, CMV, HHV6 and EBV viral loads in 83 bronchoalveolar lavage (BAL) from lung transplant recipients.
Germi et al.
Unit of Virus Host Cell Interactions, Grenoble
ESCV 2011
- Evaluation of real-time PCR for HSV-VZV on various samples in the condition of a clinical virology laboratory
ESCV 2008
- Development of a new diagnostic tool for the quantitative detection of HSV-1, HSV-2, AND VZV DNA
Vignoles et al.
ARGENE
CVS 2008
- Evaluation of Argene's Research Use Only, Real-Time PCR Reagents for HSV1 and HSV2 R-GENE
Patel et al.
Le Bonheur Children's Medical Center, Memphis.