Parvovirus B19 R-gene®
A real-time PCR kit for the detection and quantification of Parvovirus B19 DNA
- Accurate and equal quantification of 3 Parvovirus B19 genotypes over a wide linear range
- Ready-to-use kit including internal control and quantification standards
- CE-IVD on all major extraction platforms and real-time PCR systems and on different samples types
Do you need more information
Parvovirus B19 R-GENE® advantages
Infection with Parvovirus B19 can lead to serious consequences for vulnerable patients (pregnant women, patients suffering from congenital anaemia) or immunosuppressed patients (for example: transplant patients, AIDS patients, etc.). Optimized detection of Parvovirus B19 infection is therefore important. Parvovirus R-GENE® is an ideal solution that offers rapid and specific detection and quantification of the 3 Parvovirus B19 genotypes. Plus – for added efficiency and comprehensiveness – you can use most kits from the broad R-GENE® range to detect various pathogens from one sample or analyze various samples for one virus simultaneously.
- Sensitive and reproducible
- Reliable detection of Parvovirus infection
- Wide linear range
- Standardized
- Parallel processing with R-GENE® range of products (CMV R-GENE®, EBV R-GENE®, HSV1 HSV2 VZV R-GENE®, CMV HHV6,7,8 R-GENE®, BK Virus R-GENE®, ADENOVIRUS R-GENE®, CMV R-GENE®)
- Harmonized test profiles for multiple assays in one run
- Protocol to convert quantification results in IU/mL with the WHO 2nd International Standard
- Flexible:
- Validated for use with various samples types
- Prepare samples manually or use automated sample preparation, for example, NucliSENS® easyMAG® and assay setup techniques such as easySTREAM™* liquid handling platform
- Qualified with the major real-time PCR platforms
*Coming soon
Everything you require in one kit
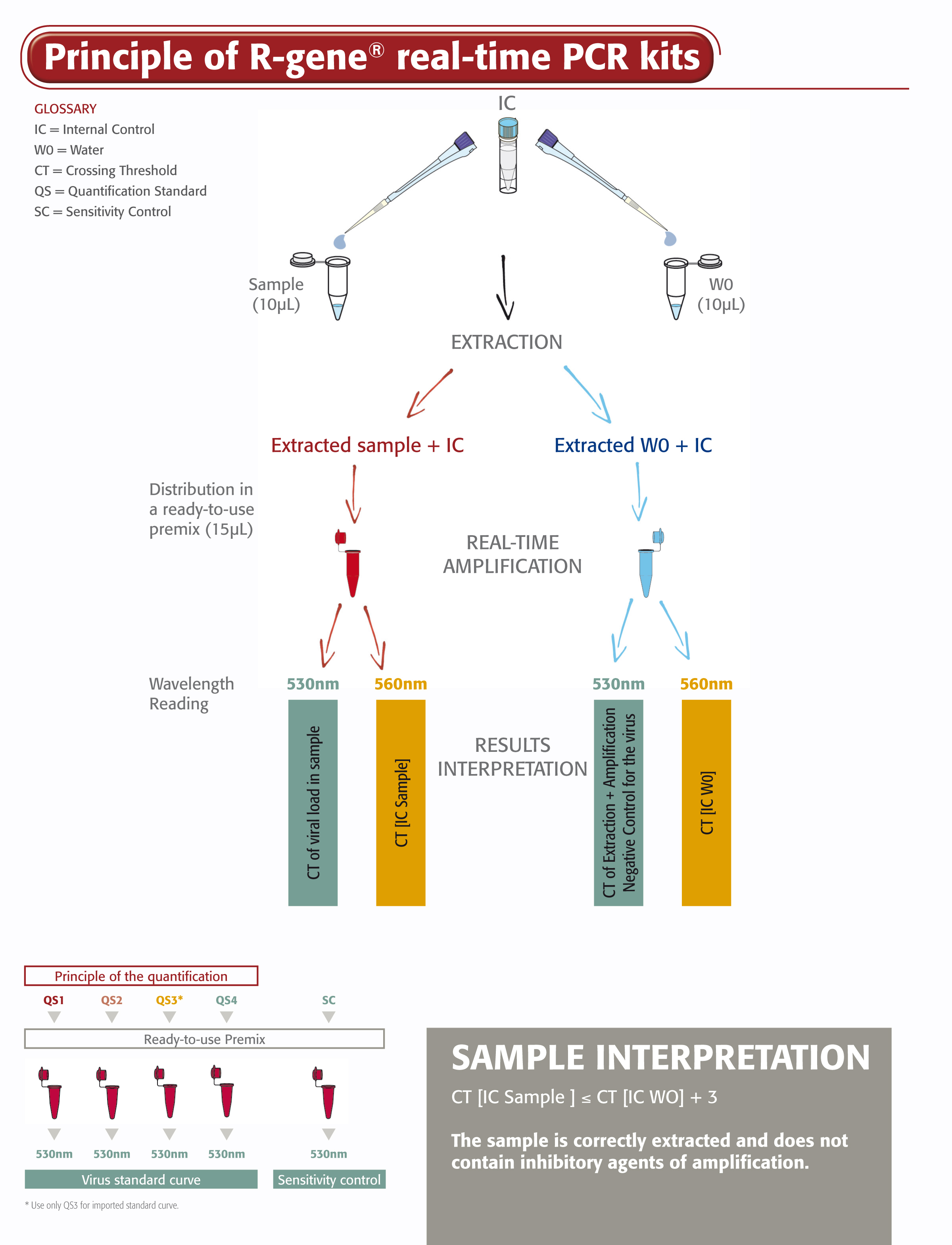
The Parvovirus B19 R-GENE® kit is a ready-to-use molecular detection kit. It measures the viral load of Parvovirus B19 using real-time PCR after viral DNA extraction from whole blood, plasma and serum and it enables the qualitative detection on bone marrow and medullar plasma. This 5’ nuclease-based real-time PCR assay amplifies a specific region of Parvovirus B19 genome.
- Four Quantification Standards ensure accurate Parvovirus B19 viral load measurement
- Sensitivity Control validates the performance of the assay
- An Internal Control (IC2) checks the extraction process, including lysis, and the presence of amplification inhibitors in the sample
- Includes all necessary reagents optimized to detect and quantify Parvovirus B19 for in vitro diagnostic use
Easy procedure
The Parvovirus B19 R-GENE® kit is easy to use. Just add the extracted DNA sample to the ready-to-use PCR master mix, then start the reaction on the appropriate Real-Time PCR thermo-cycler, following optimized cycling program described in the “Instructions For Use”.
Click to enlarge »
BIOMERIEUX, the blue logo, ARGENE®, R-GENE®, EASYMAG® and NUCLISENS® are used, pending and/or registered trademarks belonging to bioMérieux, or one of its subsidiaries, or one of its companies. Any other name or trademark is the property of its respective owner.
| Parvovirus B19 R-GENE® (69-019B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of Parvovirus B19 |
| Ordering information | Reference 69-019B: Parvovirus B19 R-gene® Detection and Quantification Kit Also available under reference 69-019: Detection and Quantification Kit COMPLETE kit (includes 69-019B Detection and Quantification Kit kit and ref. 67-000 DNA Extraction kit) |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| Gene target | NS1 gene |
| Specimen* | Whole blood, plasma, serum, bone marrow, medullar plasma |
| Limit of Detection | Whole blood: 47 Copies / mL Plasma: 70 Copies / mL |
| Dynamic Range of Quantification | For the plasma matrix: between 5.05 1010 copies/mL (i.e. 10.7 log10 cp/mL) and 87 copies/mL (i.e. 1.96 log10 cp/mL). For the whole blood matrix: between 6.1 1010 copies/mL (i.e. 10.7 log10 cp/mL) and 42 copies/mL i.e. 1.61 log10 cp/mL). |
| Controls included | Extraction + Inhibition control, Sensitivity control, negative control |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL – IU/mL (using WHO International Standard Parvovirus B19) |
| Number of tests | 90 tests |
| Storage conditions | -18°C/-22°C for reference 69-019B (Detection and Quantification Kit), +2°C/+8°C for ref. 67-000 (DNA Extraction kit) |
| Validated Extraction system* Manual Automated | QIAamp DNA Blood Mini kit NUCLISENS® EASYMAG® MagNA Pure Compact MagNA Pure LC EZ1 Advanced XL |
| Validated Amplification platform* | Life Technologies (ABI7500, ABI7500 Fast & StepOne) LightCycler 2.0 LightCycler 480 System II SmartCycler 2.0 Rotor Gene Q and Rotor Gene 6000 DX Real-Time system CFX96 |
| Status | For in vitro diagnostic use, CE marking in Europe- Please enquire |
Fast facts on Parvovirus B19
What is Parvovirus B19?
Parvovirus B19 is a member of the Parvoviridae family and the Erythrovirus genus, as it can infect erythroid precursor cells. It is a unenveloped, single-stranded DNA virus of about 5.5 kb, divided into three genotypes. The most common, Genotype 1, is found all over the world; genotypes 2 and 3 are less prevalent and more geographically restricted (Western Europe, USA, Brazil and Africa). Parvovirus B19 is mainly disseminated by aerosol or respiratory secretions.
Who is most at risk?
The virus is most associated with causing disease in the pediatric population, though it also affects adults. Epidemics generally take place at school, mainly in spring and winter. Although it is mostly asymptomatic, the primary infection can cause a benign skin rash called epidemic megalerythema or fifth disease. The most common symptom in infected adults is arthropathy. Parvovirus B19 can lead to serious consequences for vulnerable or immunodepressed patients. In pregnant women, a primary infection may lead to fetal anemia – potentially causing hydrops foetalis, spontaneous abortion or fetal death in utero. In people suffering from constitutional hemolytic anemia it can cause acute attacks of deglobulisation. In immunodepressed patients, such as AIDS or transplant patients, it may be responsible for severe chronic anemia with destruction of erythroblasts in the bone marrow. It is also associated with heart disease (myocarditis) and neurological diseases.
What are the benefits of Parvovirus B19 molecular testing?
Real-Time PCR-based assays for Parvovirus B19 enable rapid and specific detection of the virus to improve patient management. Testing helps keep track of the effectiveness of active treatment and can monitor for relapse after treatment.
Parvovirus B19 R-GENE® and the WHO International Standard for Parvovirus B19
Need to calculate your conversion factor to express your Parvovirus R-gene® results in IU/ml? Please download a calculation protocol.
Parvovirus B19 R-GENE®: POSTERS
- Development of a new diagnostic tool for the detection and quantification of Parvovirus B19 by Real Time PCR
Patricia Marechal et al. bioMérieux. ECV 2013 - Evaluation Of a Novel Real Time PCR Assay For Parvovirus B19 (B19V) Genome Detection And Quantification
Aurélie Schnuriger et al. Bacteriology-Virology laboratory, AP-HP Hôpital Trousseau Paris- France. ECV 2013