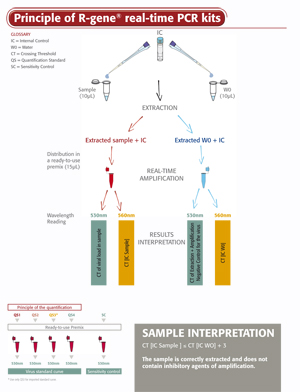
EBV R-GENE®
A real-time PCR kit for accurate detection and quantification of Epstein-Barr virus (EBV) DNA
- Accurate EBV viral load quantification over a wide linear range*
- Kit is ready to use including internal control & quantification standards
- CE-IVD on various sample types as well as on all major extraction platforms & real-time PCR systems
Do you need more information
EBV R-GENE® advantages
Epstein-Barr virus (EBV), known as the etiologic agent of infectious mononucleosis, is responsible for lymphoproliferation of B cells (Post-Transplant Lymphoproliferative Disorder (PTLD), Burkitt’s Lymphoma, Hodgkin’s lymphoma, …), a serious complication for immunocompromised patients. Optimized detection and monitoring of EBV infection is critical to ensure the best patient management. EBV-R-GENE® offers rapid and specific detection even prior to clinical symptoms, making it an ideal solution. This helps improve options for management, measure the effectiveness of treatment, and monitor for relapse. For even more comprehensive testing, you can use the broad R-GENE® range to quantify different viruses in one sample or analyze various samples for one virus at the same time.
- Sensitive and reproducible
- Reliable measurement of EBV infection
- Wide linear range
- Standardized
- Uniform processing with R-GENE® range of products (CMV R-GENE®, HSV1 HSV2 VZV R-GENE®, CMV HHV6,7,8 R-GENE®, BK Virus R-GENE®, Adenovirus R-GENE®, Parvovirus B19 R-GENE®) Harmonized test profiles for multiple assays in one run
- Protocol to convert quantification results into IU/mL with the WHO 1st International Standard
- Flexible
- Validated for use with various sample types
- Use manual or automated sample preparation such as NucliSENS® easyMAG® and assay setup system such as easySTREAM™ liquid handling platform
- Qualified with the major real-time PCR platforms
Everything you need in one kit
The ready-to-use EBV R-GENE® molecular detection kit measures EBV viral load in DNA extracts from different clinical samples. This 5’ nuclease-based real-time PCR assay amplifies a specific region of the EBV genome for detection and quantification of EBV.
- Four Quantification Standards ensure accurate EBV viral load measurement
- Sensitivity Control validates the performance of the assay
- An Internal Control (IC2) checks the extraction process, including lysis, and the presence of amplification inhibitors in the sample incredibly
- All necessary reagents optimized to detect and quantify EBV for in vitro diagnostic use are ready to use:
- Less technician time
- Less risk of manipulation or dilution error
- Less risk of contamination
Easy procedure
Using the EBV R-GENE® kit is so simple, all you need to do is add the sample extracted DNA to the ready-to-use PCR master mix and start the reaction on the appropriate Real-Time PCR thermocycler, following the optimized cycling program described in the “Instructions For Use”.
Click to enlarge »
BIOMERIEUX, The blue logo, ARGENE®, R-GENE®, EASYMAG® and NUCLISENS® are used, pending and/or registered trademarks belonging to bioMérieux, or one of its subsidiaries, or one of its companies. Any other name or trademark is the property of its respective owner.
Any other name or trademark is the property of its respective owner. - See more at: http://clinical.biomerieux.net/cmv-R-GENE-0#sthash.YR9WVorN.dpuf
Any other name or trademark is the property of its respective owner. - See more at: http://clinical.biomerieux.net/cmv-R-GENE-0#sthash.YR9WVorN.dpuf
Any other name or trademark is the property of its respective owner. - See more at: http://clinical.biomerieux.net/cmv-R-GENE-0#sthash.YR9WVorN.dpuf
| EBV R-GENE® (69-002B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of EBV |
| Ordering information | Reference 69-002B : EBV R-GENE® Quantification kit Also available under reference 69-002 : EBV R-GENE® Quantification COMPLETE kit (included 69-002B Quantification kit and ref. 67-000 DNA extraction kit) |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| Gene target | BXLF1 gene coding for thymidine kinase |
| Specimen* | Whole blood, plasma, CSF, biopsies, BAL |
| Limit of Detection | 4 copies/PCR |
| Dynamic Range of Quantification | Up to 5 106 copies/ mL |
| Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL or convert to IU/mL with WHO 1st International |
| Standard | |
| Number of tests | 90 tests |
| Storage conditions | -18°C/-22°C for reference 69-002B (Quantification kit) +2°C/+8°C for ref. 67-000 (DNA extraction kit) |
| Validated Extraction platform* Manual Automated | QIAamp DNA Blood Mini kit NucliSENS® easyMAG® MagNA Pure Compact MagNA Pure LC QIAcube QIAsymphony SP m2000sp VERSANT kPCR Molecular System SP |
| Validated Amplification platform* | Life Technologies (Applied Biosystems) LightCycler SmartCycler RotorGene VERSANT kPCR Molecular System AD Biorad DX Real-Time system Stratagene / Agilent |
| Status | For in vitro diagnostic use |
* please enquire
Fast facts on EBV
What is EBV?
Epstein-Barr (EBV) virus is a ubiquitous herpesvirus which infects the majority of human population. After a primo infection (transmission by saliva), EBV remains latent in B cells and can periodically reactivate in healthy individuals. This reactivation occurs with a replication of EBV in the oropharynx and does not have any clinical signs.
Who is most at risk?
EBV is a causative agent for a variety of clinical conditions including infectious mononucleosis (IM) and several pathogenesis of cancers such as Burkitt's lymphoma, Hodgkin's lymphoma, and nasopharyngeal carcinoma. In immunosuppressed individuals, uncontrolled EBV-induced proliferation of B-cells results may also lead to post-transplant lymphoproliferative disorder (PTLD), which is an important cause of morbidity and mortality in both solid organ transplant recipients and HSCT patients.
What are the benefits of EBV molecular testing?
Real-Time PCR-based assays for EBV enable rapid and specific detection prior to clinical symptoms to help improve outcomes. This is especially important for immunosuppressed patients who are at risk of particularly severe consequences of infection. Quantitative EBV Real-Time PCR can be used to aid in the early diagnosis of PTLD, track the course of the disease, and monitor response to treatment.
EBV R-GENE® and the 1st WHO International Standard for Epstein-Barr (EBV) virus
Need to calculate your conversion factor to express your EBV R-GENE® results in IU/ml?
Please download a calculation protocol.
R-GENE®: PUBLICATIONS
- Evaluation of the Epstein-Barr virus R-GENE quantification kit in whole blood with different extraction methods and PCR platforms
Fafi-Kremer et al.
J Mol Diagn. 2008 Jan;10(1):78-84. Epub 2007 Dec 28
- Measurement of HSV1, CMV, HHV6 and EBV viral loads in 83 bronchoalveolar lavage (BAL) from lung transplant recipients.
Germi et al.
Unit of Virus Host Cell Interactions, Grenoble
ESCV 2011
- Evaluation de la trousse EBV R-GENE (Argene) : Mesure de la charge virale EBV dans des prélèvements de sang total et dans les échantillons du panel européen QCMD
Brunet et al.
Service de virologie, Groupe Hospitalier Pitié-Salpêtrière, AP-HP, Paris
RICAI 2010
- Intérêt de la PCR quantitative EBV dans le suivi des greffés de moelle osseuse en pédiatrie
Billaud et al.
Laboratoire de Virologie, Hopital Debrousse, Lyon.
RICAI
- Comparative evaluation of the NucliSENS® easyMAG™ automated system for the extraction of viral DNA from whole blood samples: application to the monitoring of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) loads
Pillet et al
Laboratoire de Bacteriologie-Virologie-Hygiène, Hopital Nord, CHU de Saint-Etienne, France.
ECCMID 2008
- Clinical and Laboratory Evaluation of the new Argene EBV DNA quantitative real-time PCR assay (R-GENE)
Laura Jane Scott et al
West Scotland Specialist Virology Centre, Gartnavel General Hospital, Glasgow, Scotland.
International Symposium on molecular diagnostics, 2006