Adenovirus R-GENE®
A real-time PCR kit for the detection and quantification of Adenovirus DNA
- Accurate quantification of Adenovirus viral load over a wide linear range
- Detection of all know serotypes
- Ready-to-use kit that includes internal control and quantification standards
- CE-IVD on different samples types and on all major extraction platforms and real-time PCR systems
Do you need more information
Adenovirus R-GENE® advantages
Adenovirus is a serious complication for immunocompromised patients, leading to high levels of morbidity and mortality. Optimized detection and monitoring of Adenovirus infection is therefore very important for improved patient management. Providing detection and/or quantification of the genome of all known adenovirus serotypes, Adenovirus R-GENE® is an ideal solution. It offers rapid and specific detection to help you track the course of infection and monitor response to treatment. To further improve the comprehensiveness of testing, most of the R-GENE® range lets you quantify various pathogens in one sample or analyze various samples for one virus at the same time.
- Sensitive and reproducible
- Reliable measurement of Adenovirus infection
- Wide linear range
- Standardized
- Harmonized test profiles for multiple assays in one run
- Uniform processing with R-GENE® range of products (CMV R-GENE®, HSV1 HSV2 VZV R-GENE®, CMV HHV6,7,8 R-GENE®, BK Virus R-GENE®, EBV R-GENE®, Parvovirus B19 R-GENE®, BORDETELLA R-GENE®)
- Flexible
- Validated for use with various samples types
- Prepare samples manually or use automated sample preparation such as NucliSENS® easyMAG® and assay setup techniques such as easySTREAM™
- Qualified with the major real-time PCR platforms
All you need in one kit
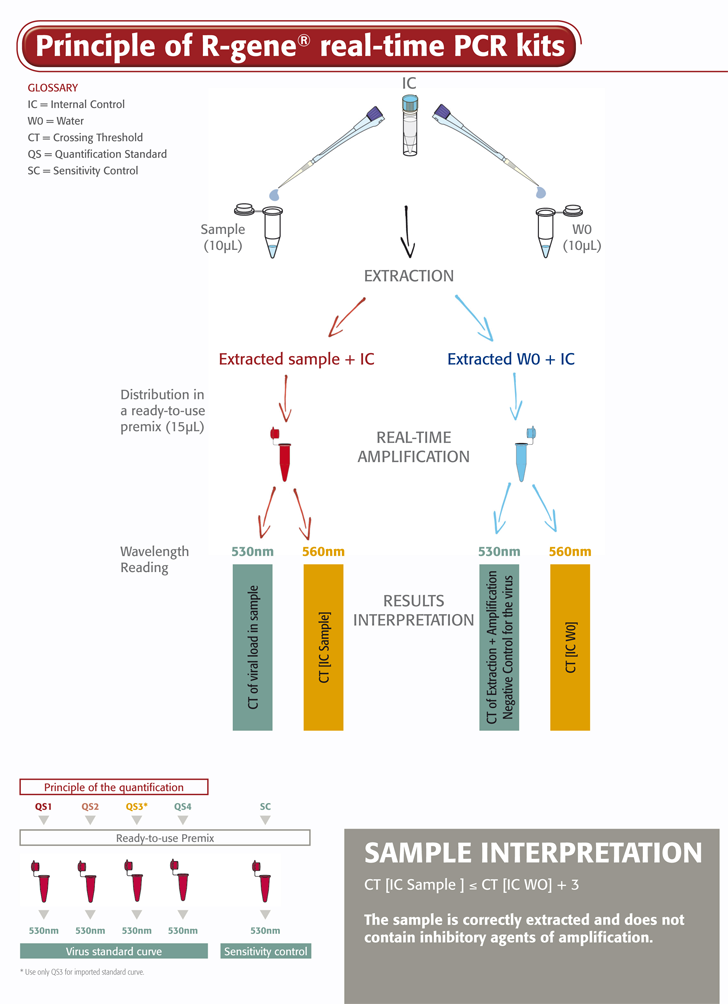
The Adenovirus R-GENE® kit is a ready-to-use molecular assay designed to detect and quantify the viral load of Adenovirus using real-time PCR after viral DNA extraction. A 5’ nuclease-based assay, it amplifies a specific region of the Adenovirus genome.
- Four Quantification Standards ensure accurate Adenovirus viral load measurement
- Sensitivity Control validates the performance of the assay in the lab routine
- An Internal Control (IC2) checks the extraction process, including lysis and the presence of amplification inhibitors in the sample
- Includes all necessary reagents optimized to detect and quantify Adenovirus for in vitro diagnostic use
Easy procedure
The Adenovirus R-GENE® kit is simple to use. Just add the extracted DNA sample to the ready-to-use PCR master mix and start the reaction on the appropriate Real-Time PCR thermocycler, following the optimized cycling program described in the “Instructions For Use”.
BIOMERIEUX, the blue logo, ARGENE®,R-GENE®, EASYMAG® and NUCLISENS® are used, pending and/or registered trademarks belonging to bioMérieux, or one of its subsidiaries, or one of its companies.
Any other name or trademark is the property of its respective owner.
| Adenovirus R-GENE® (69-010B) | |
|---|---|
| Principle of the test | Genomic detection and quantification of Adenovirus |
| Ordering information | Reference 69-010B: Adenovirus R-GENE® Detection and quantification kit Also available under reference 69-010: Adenovirus R-GENE® Detection and quantification COMPLETE kit (include 69-010B Quantification kit and ref. 67-000 DNA Extraction kit) |
| Technology | Real-Time PCR / 5‘ nuclease Taqman technology |
| Gene target | Hexon gene |
| Specimen* | Whole blood, plasma, CSF, Stool, biopsies, Ocular specimen, Respiratory samples (Nasopharyngeal aspirates, BAL, nasal swabs) |
| Limit of Detection | Whole blood: 8 copies/PCR (200 copies/ mL) Nasal specimen: 10 copies/PCR (261 copies/ mL) Whole blood: 550 copies/mL Nasal secretions: 670 copies/mL |
| Dynamic Range of Quantification | Whole blood: 2.58 103 copies/mL to 4.65 1010 copies/mL Nasopharyngeal sample: 5.19 103 copies/mL to 8.25 1010 copies/mL Up to 5X106 copies/ mL |
| Controls included | Extraction + Inhibition control, Sensitivity control, Negative control |
| Results within | 75 minutes (extraction step not included) |
| Reporting unit | Copies/mL |
| Number of tests | 90 tests |
| Storage conditions | -18°C/-22°C for reference 69-010B (Quantification kit) +2°C/+8°C for ref. 67-000 (DNA Extraction kit) |
| Validated Extraction platform* | |
| Manual | QIAamp DNA Blood Mini kit QIAamp DNA Stool Mini kit QIAcube |
| Automated | NucliSENS® easyMAG® QIAsymphony MagNA Pure LC MagNA Pure Compact MagNA Pure 96 Biorobot M48 m2000sp VERSANT kPCR Molecular System SP |
| Validated Amplification platform* | Life Technologies (ABI7500, ABI7500 Fast & StepOne) LightCycler 1.0 LightCycler 2.0 LightCycler 480 II SmartCycler 2.0 RotorGene VERSANT kPCR Molecular System AD Biorad DX Real-Time system Stratagene / Agilent |
| Status | For in vitro diagnostic use, CE marking in Europe |
* please enquire
Fast facts on Adenovirus
What is Adenovirus?
Adenoviruses are medium-sized, double-stranded DNA, non-enveloped icosahedral viruses. At least seven human adenoviruses species (A-G), including 52 serotypes, have been described. Adenoviruses can cause respiratory, ocular or gastrointestinal disease mainly occurring in children and military recruits as endemic infections or during outbreaks. Adenovirus infections are common and occur throughout the year, around the world.
Who is most at risk?
Over the last few years, adenoviruses have been increasingly recognized as significant viral pathogens with high morbidity and mortality among immunocompromised patients. Clinical manifestations in immunocompromised patients include pneumonia, hepatitis, hemorrhagic cystitis, colitis, pancreatitis, meningoencephalitis and disseminated disease, depending on the underlying disease, affected organ system, patient age, and virus serotype.
What are the benefits of adenovirus testing?
Real-Time PCR-based assays for adenovirus enable rapid and specific detection prior to clinical symptoms to help improve outcomes, which is especially important for immunosuppressed patients, who can be severely affected. Testing helps keep track of the effectiveness of active treatment and can be used to monitor for relapse after treatment.
Adenovirus R-GENE®: PUBLICATIONS
- Comparison of the EZ1 XL advanced and the Magna Pure instruments for the extraction of whole blood before DNA quantification of CMV, EBV, HHV 6 and Adenovirus
Marie Gueudin, Alexandre Louvel, Jean-Christophe Plantier, Laboratoire de virologie, CHU de Rouen, Université de Rouen, France. ECV 2013 - Comparison of in-house real-time quantitative PCR to the Adenovirus R-GENE kit for determination of adenovirus load in clinical samples.
Jeulin et al. Laboratory of Virology, Nancy University Hospital, France. J Clin Microbiol. 2010 Sep;48(9):3132-7. Epub 2010 Jul 14. - Adenoviruses in Immunocompromised Hosts
Echavarria. Laboratory of Clinical Virology, University Hospital, Buenos Aires. Clinical Microbiology Reviews, Oct 2008, 704-715 - Analytical and Clinical Evaluation of the Argene Adenovirus R-GENE Assay.
Malgorzata Kowerska et al. Department of Pathology and Laboratory Medicine, North Shore-LIJ Health System Laboratories, NY. CVS 2011 - Prognostic value of qPCR for Adenovirus detection in stool samples compared with antigen detection and cell culture in hematopoietic cell transplant recipients and evaluation of Adenovirus R-GENE™ kit
Jeulin et al. Laboratory of Virology, Nancy University Hospital, France. CVS 2010 - Outbreak of Adenovirus keratoconjuntivitis in health care workers : coinfection with different Adenovirus species.
Echavarria et al. Laboratory of Clinical Virology, University Hospital, Buenos Aires. CVS 2009 - Development of a new diagnostic tool for the quantification of Adenoviruses by Real Time PCR.
Magro et al. ARGENE. ESCV 2010 - A commercial Real Time PCR for Adenovirus
Echavarria et al. Laboratory of Clinical Virology, University Hospital, Buenos Aires. ESCV 2009 - Validation of commercial (ARGENE) FLU A/B, RSV A/B and Adenovirus PCR assay.
Hocker et al. Scott&White Hospital/Texas A&M Health Science Center College of Medicine, Temple, Texas, USA. CVS 2009 - Detection of Adenovirus DNA from Clinical specimens using commercial Real Time PCR reagents.
Zheng et al. Children's Memorial Hospital, Northwestern University, Chicago, Illinois. - Real-time PCR for the detection of respiratory viruses.
Seffar et al. Molecular Diagnostic Center, Laboratory of Virology, Erasme Hospital, Brussel, Belgium. ESCV 2009 - H.JEULIN, A.SALMON, P.BORDIGONI and V.VENARD
Comparaison of In-house Real-Time Quantitative PCR to the Adenovirus R-GENE Kit for Determination of Adenovirus Load in Clinical Samples
Journal of Clinical Microbiology, Sept.2010.p.3132-3137 - TATE JE, BUNNING ML, LOTT L, LU X, SU J, METZGAR D., BROSCH L., PANOZZO CA, MARCONI VC, FAIX DJ, PRILL M, JOHNSON B, ERDMAN DD, FONSECA V, ANDERSON LJ, WIDDOWSON MA
Outbreak of Severe Respiratory Disease Associated with Emergent Human Adenovirus Serotype 14 at a US Air Force training Facility in 2007.
J Infect Dis. 15;199(10):1419-1426, May 2009 - ECHAVARRIA M.
Adenoviruses in immuno compromised Host. Clinical Microbiology reviews, Oct 2008, p.704-715. - VABRET A., GOUARIN S., JOANNES M., BARRANGER C., PETITJEAN J., CORBET S., BROUARD J., DUHAMEL JF., GUILLOIS B. AND FREYMUTH F.
Development of a PCR and hybridization-based assay (PCR ADENOVIRUS CONSENSUS) for the detection and the species identification of adenoviruses in repiratory specimens.
J.Clin virology 31(2):116-22, Oct 2004. - HEIM A., EBNET C., HARSTE G., PRING-AKERBLON P.
Rapid and Quantitative Detection of Human Adenovirus DNA by Real-Time PCR.
Journal of Medical Virology 70: 228-239, 2003.