VIDAS® EBV panel
Diagnosis of Infectious Mononucleosis
The VIDAS® Epstein-Barr Virus (EBV) panel provides a combination of three tests to rapidly confirm a diagnosis of Infectious Mononucleosis and clearly establish the stage of infection.
- 4 EBV-specific markers in 3 tests for a confident diagnosis
- Flexible solution adapted to small-volume testing
- Automated diagnosis you can perform in your own lab to provide same-day results to clinicians
- All-inclusive kits
Do you need more information
Through detection of the main causative agent of Infectious Mononucleosis (IM), the VIDAS® EBV panel helps confirm a clinical diagnosis of the disease and rule out other pathologies which may produce IM-type symptoms. Rapid and clear diagnosis enables to reassure patients and physicians and avoid unnecessary investigations and treatment.
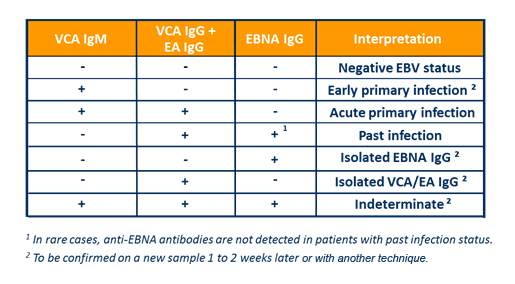
The panel includes three automated tests for the qualitative detection of anti-VCA IgM, anti-EBNA IgG and anti-VCA IgG/anti-EA IgG antibodies in human serum using the ELFA technique. Based on proprietary peptides, these assays are designed to provide the most relevant antibody responses to confidently define patient status.
The full serological picture
- Rational criteria to define EBV patient status in immunocompetent individuals
|
VCA = Viral Capsid Antigen |
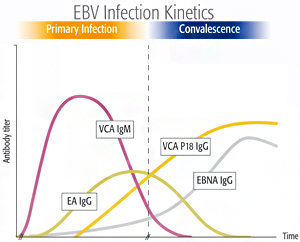
The VIDAS® EBV-specific tests are related to the time-dependent antibody response to various antigens produced during the Epstein-Barr Virus life cycle.
3 serological parameters - VCA IgM, VCA IgG and EBNA IgG – are necessary for EBV stage-specific diagnosis2,3:
- Primary infection
- Past infection
- Absence of infection
- EBV serology for confident diagnosis
- More reliable than Monospot or heterophile antibody testing1,2*
- Allows to definitively confirm or exclude Infectious Mononucleosis in most cases**
* See resources for more information
** Diagnosis is established taking into account the age of the patient and the complete clinical picture.
- Combined EA IgG and VCA IgG detection for quicker confirmation
Since 80% of patients yield EA IgG during acute IM, the combination of two markers in one test enables confirmation of the disease through earlier IgG detection.
- Easy interpretation
References:
(1) Macsween KF and Crawford DH. Epstein-Barr virus – recent advances. Lancet Infectious Diseases. 2003;3:131-140.
(2) Hess RD, et al. Routine Epstein-Barr Virus Diagnostics from the Laboratory Perspective: Still challenging after 35 years. J Clin Microbiology 2004;42:3381-3387.
(3) Lupo J, et al. Performance of Two Commercially Available Automated Immunoassays for the Determination of Epstein-Barr Virus Serological Status. Clin. Vaccine Immunol. 2012, 19(6):929. DOI: 10.1128/CVI.00100-12.
Test benefits
Flexible and easy-to-use
- Fully automated EBV testing
- Just load & go
- 24/7 availability
- Both on-demand and routine testing possible
Rapid
- Results within 40 minutes for the 3 VIDAS® EBV tests
Cost-effective
- Standards and controls included in the kit
- Only 30 tests / kit
- Calibration once every 28 days
| VIDAS® VCA IgM | VIDAS® VCA/EA IgG | VIDAS® EBNA IgG | |
|---|---|---|---|
| Reference | 30 237 | 30 236 | 30 235 |
| Tests / kit | 30 | 30 | 30 |
| Time to result | 40 minutes | 40 minutes | 40 minutes |
| Sample type | Serum | Serum | Serum |
| Sample volume | 100 µL | 100 µL | 100 µL |
| Calibration frequency | Every 28 days | Every 28 days | Every 28 days |
Find more technical details on www.myvidas.com.
Consult your local bioMérieux representative for product availability in your country
Related Publications
- Lupo J, et al. Performance of Two Commercially Available Automated Immunoassays for the Determination of Epstein-Barr Virus Serological Status. Clin. Vaccine Immunol. 2012, 19(6):929. DOI: 10.1128/CVI.00100-12.
- Koidl C., et al. Performance of new enzyme-linked fluorescent assays for detection of Epstein-Barr virus specific antibodies in routine diagnostics. Wien KlinWorchenschr. 2011 DOI 10.1007/s00508-011-1561-z
- Determination of EBV serostatus prior to kidney transplantation: Comparison of VIDAS(®), LIAISON(®) and immunofluorescence assays. Johannessen I, Noel M, Galloway A, Black S, Shearman M, Graham C. J Virol Methods. 2014 Jul;203:107-11.
- Johannessen I, et al. Determination of EBV serostatus prior to kidney transplantation: Comparison of VIDAS®, LIAISON® and immunofluorescence assays. J. Virol. Methods (2014)
EBV Serology vs Heterophile Antibodies
The Monospot (or heterophile antibody) tests detect antibodies which, although not directed against EBV, are highly indicative of IM when present. Some have an excellent positive predictive value, but the method has limitations1,2:
|
=> A negative test cannot exclude IM. |
As a result, EBV serology is the method of choice for the diagnosis of IM.
References:
Educational Resources
Infectious Mononucleosis: 10 Questions & Answers
Find more scientific and educational resources on www.myvidas.com.